- Homepage
- MDIS MarketPlace
- DHF Report
Polarion Marketplace
Explore top Polarion custom use cases showcasing real-world business transformation and process innovation for customers.

Design History File Report
By Maqsusi Digital Industries Software
For Siemens Polarion ALM.
Overview
- The Automated Design History File (DHF) Compilation System is a customized solution developed within Polarion ALM to streamline and automate the generation of regulatory-compliant design documentation. This customization ensures full alignment with Quality Management System (QMS) standards and global regulatory frameworks such as ISO 13485 and FDA 21 CFR Part 820.
- The system automatically aggregates all essential project artifacts — including design inputs, design outputs, verification and validation records, approvals, and traceability matrices — into a single, structured DHF report. By integrating Polarion’s native linking and workflow capabilities, the solution provides end-to-end visibility into the product development lifecycle, ensuring that every design change, review, and approval is properly captured and auditable.
- Through centralized data access, the system eliminates manual document collection and version tracking efforts, significantly reducing the risk of human error. It maintains documentation integrity, supports eSignatures and review tracking, and provides version-controlled audit trails to facilitate seamless regulatory inspections.
-
This DHF automation not only improves traceability and compliance readiness but also strengthens quality assurance and documentation consistency across multidisciplinary teams. Ultimately, it empowers organizations to accelerate product development while maintaining strict adherence to regulatory and quality standards.
Functinal Flow
- Artifact Collection & Data Retrieval – The system automatically gathers all relevant artifacts — including design inputs, outputs, verification results, validations, and approval records — directly from Polarion’s project repositories using its core APIs.
- Data Structuring & Traceability Mapping – Retrieved data is organized into a structured DHF format, maintaining end-to-end traceability between requirements, design elements, test cases, and validation records.
- Workflow Integration & Approval Tracking – The customization integrates with Polarion’s workflow engine to capture review comments, approval statuses, and eSignatures for each artifact in real time.
- Automated DHF Compilation & Report Generation – Once the data is aggregated, the system compiles it into a regulatory-compliant DHF report, formatted for audits and exportable in standard documentation formats (PDF, HTML, DOCX).
-
Version Control & Audit Readiness – The final DHF report is version-controlled and stored within Polarion, providing a complete audit trail for compliance checks and external inspections.
Benefits
- Regulatory Compliance Assurance – Ensures automatic alignment with standards such as ISO 13485 and FDA 21 CFR Part 820 by maintaining structured, auditable design documentation.
- Enhanced Traceability – Establishes a seamless linkage between all design and verification artifacts, ensuring full visibility throughout the product development lifecycle.
- Reduced Manual Effort – Automates the traditionally time-consuming DHF compilation process, minimizing human error and saving significant administrative effort.
- Improved Audit Preparedness – Provides a complete, version-controlled DHF ready for internal quality reviews or external regulatory audits at any time.
-
Cross-Functional Collaboration – Centralizes documentation access across engineering, quality, and regulatory teams, promoting transparency and alignment in product development activities.
Future Enchancements
- AI-Based Data Validation – Implement intelligent validation algorithms to automatically detect missing or inconsistent documentation elements before DHF finalization.
- Interactive Dashboard View – Develop a graphical DHF dashboard to visualize documentation completeness, approval progress, and compliance status in real time.
- Automated Regulatory Template Mapping – Add the capability to generate DHFs automatically formatted to meet specific regulatory body templates (e.g., FDA, EU MDR).
- Integration with External QMS Tools – Enable data exchange and synchronization between Polarion and third-party QMS or PLM systems for unified quality tracking.
-
Digital Sign-Off Workflow – Introduce enhanced eSignature workflows integrated with secure authentication for global regulatory acceptance (e.g., compliant with 21 CFR Part 11).
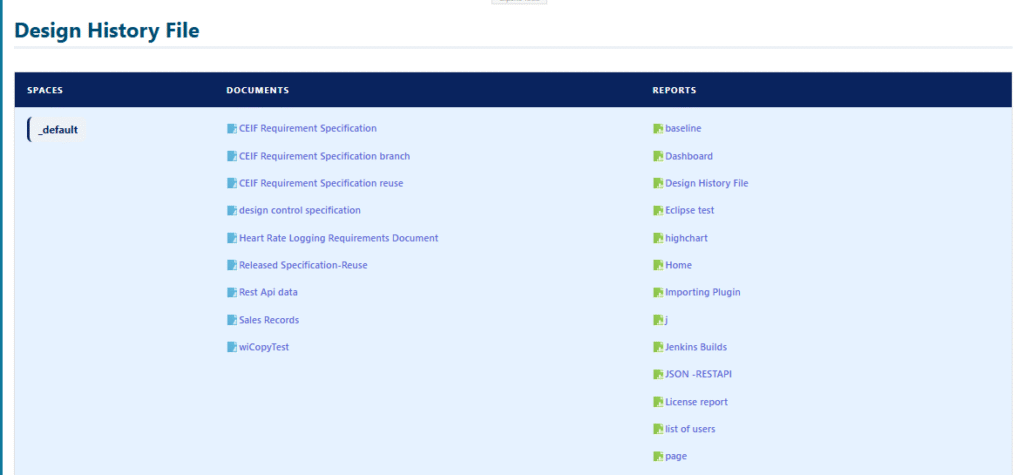
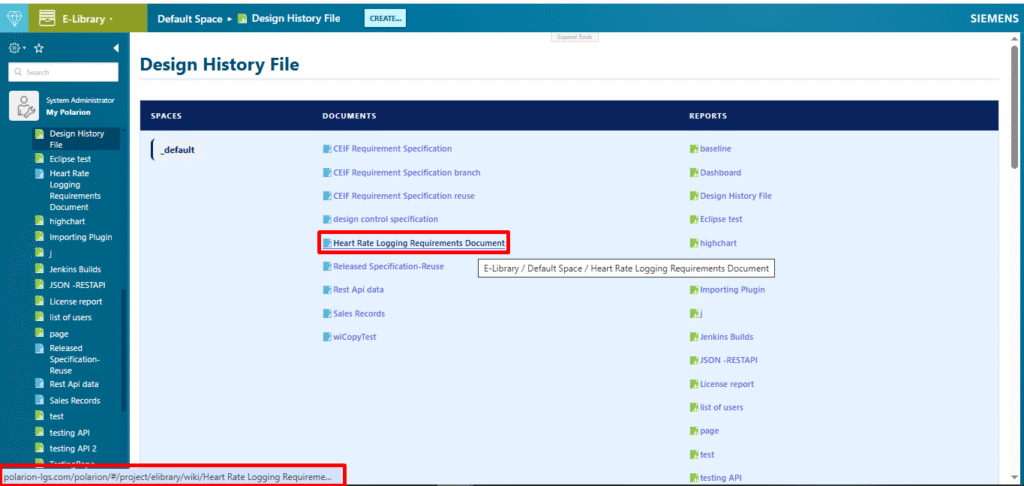
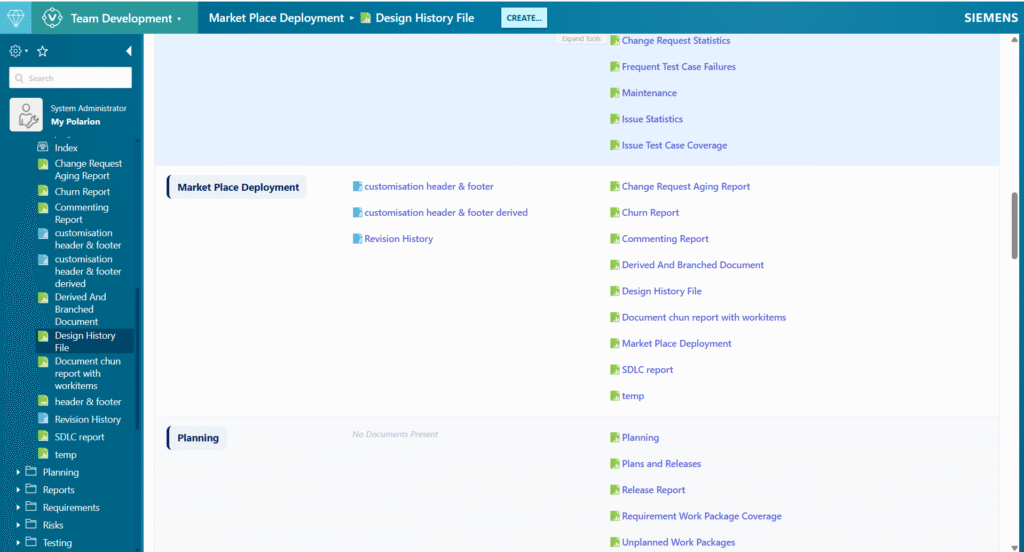
Samples
Vendor
Maqsusi Digital Industries Software
Published by
Pagilla Pavan Kalyan
Products
- Polarion ALM
- Polarion QA
- Polarion Requirements
- Polarion X
Polarion Version
2506